Worth knowing from the area of cleanroom qualification
The knowledge area cleanroom qualification answers questions about the cleanroom classes and the tests within the scope of a cleanroom qualification. You will also receive an overview of the specifications from DIN EN ISO 14644-3:2020-08.
Here you will find answers to the following questions:
- Relevant standards and guidelines
- Which cleanroom classes are there?
- Operating conditions of cleanrooms according to DIN EN ISO 14644
- Which tests/measurements are carried out during a cleanroom qualification?
- What innovations does DIN EN ISO 14644-3:2020-08 bring with it with regard to the performance of leak tests on HEPA filters?
- What changes were introduced in the August 2022 revision of Annex 1 of the EU GMP Guidance for the qualification of cleanrooms?
- Monitoring strategies CCS and 14644-2 – what can be done and what are they good for?
- Filters and filter integrity tests
Relevant standards and guidelines
The subject area of cleanrooms and cleanroom qualification is very complex, as various systems are interrelated and are responsible to varying degrees for the performance of the cleanroom. These systems or subsystems are often covered by various standards and guidelines. The following is an overview of a few important guidelines:
EU GMP Guidelines, Annex 1 (The Rules Governing Medicinal Products in the European Union
Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use)
Annex 1 of the EU GMP Guidelines regulates the requirements for the manufacture of sterile medicinal products and was comprehensively revised in 2022.
Aseptic guide (Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing — Current Good Manufacturing Practice):
The FDA guidance describes the requirements for the aseptic manufacture of sterile medicinal products in accordance with current GMP specifications. It dates from 2004 and supplements the regulatory requirements of 21 CFR Parts 210 and 211.
DIN EN ISO 14644 series of standards
ISO 14644 is an internationally recognised series of standards that specifies requirements and procedures for the classification, monitoring, testing and operation of cleanrooms and clean areas. It is particularly relevant for industries with high requirements for product and process purity.
ISO 14644-1: Classification of air cleanliness according to particle concentration
ISO 14644-2: Monitoring to confirm cleanroom performance
ISO 14644-3: Test methods
Other relevant standards and guidelines
- VDI 2083 series of standards
- VDI 6022
- DIN EN 1822-1 and DIN EN ISO 29463:1-4
- DIN EN ISO 17141 Biocontamination control
- DIN EN 12469 Biotechnology – Performance criteria for microbiological safety cabinets
- DIN EN 12599 Ventilation for buildings – Test methods and measurement procedures for the handover of ventilation and air conditioning systems
- DIN EN 12980 Laboratory equipment – Safety cabinets and isolators for cytostatic drugs and other CMR drugs
Which cleanroom classes are there?
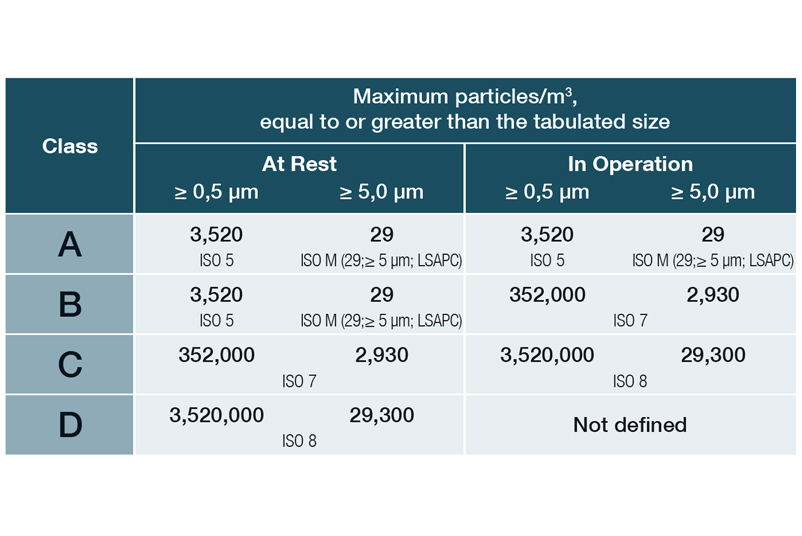
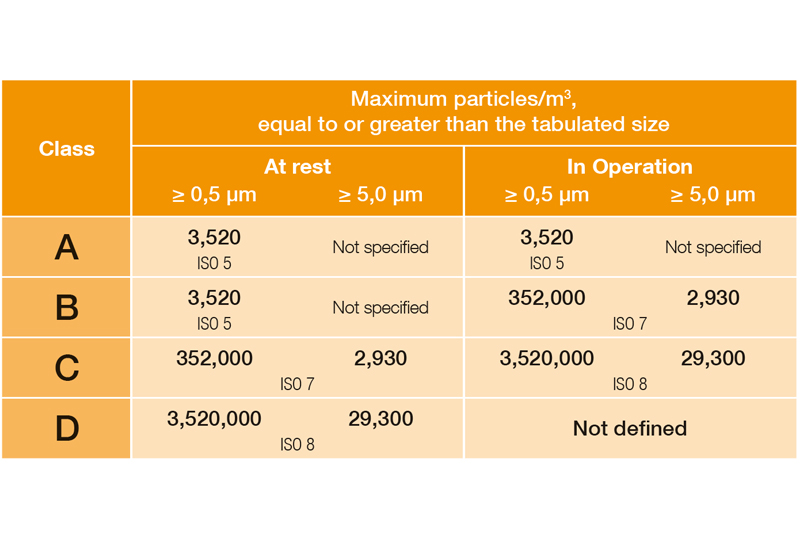
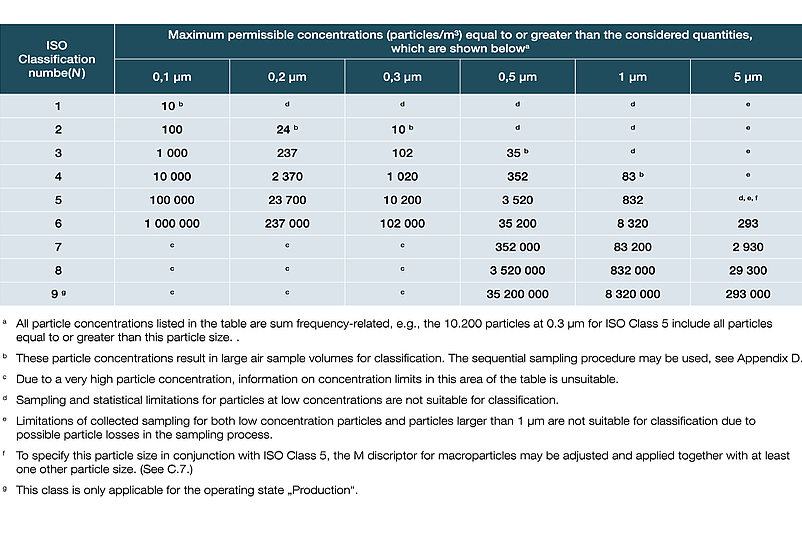
In DIN EN ISO 14644-1, the various cleanroom classes (ISO 1-9) are classified on the basis of the maximum permissible particle concentration (in particles per cubic metre of air). In the EU GMP guidelines, the cleanroom classes are assigned the letters A to D, with an additional distinction being made between dormante state and manufacturing.
Limit values for particles (permanent monitoring) in Annex 1 as of 08/2022:
EU Guidelines to Good Manufacturing Practice, Annex 1, 08/2022
Limit values classification (qualification) in Annex 1 as of 08/2022:
EU Guidelines to Good Manufacturing Practice, Annex 1, 08/2022
Classification of air purity based on particle concentrations in accordance with DIN EN ISO 14644-1:2016:
Operating conditions of cleanrooms according to DIN EN ISO 14644
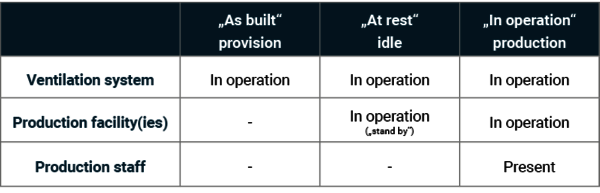
Different operating conditions are defined for the qualification and requalification of cleanrooms, particularly for determining the particulate and microbiological cleanliness class. Measurements in the different conditions ensure that potential contamination issues can be detected as early as possible in the initial qualification phase (as built). Measurements in the ‘at rest’ and ‘in operation’ states show whether the design of the cleanroom and the air flow are sufficient to reduce possible contaminants.
- ‘As built’, provision
Condition of the fully connected and operational (ventilation) system, but without production facilities, equipment or personnel. - ‘At rest’, idle
Condition of the complete (ventilation) system with built-in production facilities, operating* as agreed between the customer and supplier, but without personnel.
(*can only be on standby without personnel) - ‘In operation’, production
Condition of the system running in the specified operating mode with the intended staffing level as agreed.
Which tests/measurements are carried out during a cleanroom qualification?
The following measurements are typically performed during a cleanroom qualification:
- Climate measurements
- Pressure measurements
- Flow measurements
- Determination of the Particulate Purity Class
- Filter Integrity Tests and Measurement of Recovery Times
- Microbiological monitoring
What innovations does DIN EN ISO 14644-3:2020-08 bring with it regarding the performance of leak tests on HEPA filters?
Performing the filter leak test has not become any easier. There is additional formalism (e.g., derivation of Dp) and continued heavy fare in leakage definition via Na. The conformity statement for filters H13/H14 has become more stringent by a factor of 5. This has an impact on the feasibility (more re-measurements) and especially on the aerosol load of the HEPA filters. The concentrations increase several times compared to the previous version. The acceptance criterion of an in-situ test was formulated more strictly than the local transmittance in EN 1822-1. Further aggravation comes from the unnecessary definition of Na/Np. By choosing such a small value for counting events, there will be many counting events.
What changes were introduced in the August 2022 revision of Annex 1 of the EU GMP Guidance for the qualification of cleanrooms?
Despite some changes in the August 2022 version of Annex 1 of the EU GMP Guidance, the basic concept remains unchanged. There are no significant alterations to the recovery time definition, measurement of air velocities in the TAV area, and microbial status. However, a contamination control strategy (CCS) is now required as a central element of quality assurance measures. Additionally, specific requirements for minimum efforts and intervals for qualification measurements are introduced, with an explicit distinction between measurements in the "at rest" and "in operation" operating states. The harmonization of limit values for particle concentration in determining the particulate cleanliness class with DIN EN ISO 14644-1:2016 creates a discrepancy between the now different measures of qualification and monitoring.
Establishment of a GMP monitoring concept in the pharmaceutical manufacturing sector
We support you with our services in this area - find out more!
Monitoring strategies CCS and 14644-2 – what can be done and what are they good for?
A cleanroom monitoring strategy is a structured, risk-based approach to continuously assessing environmental conditions with the aim of ensuring cleanroom performance and identifying contamination risks at an early stage.
The practical implementation of DIN EN ISO 14644-2:2016 is ushering in a paradigm shift: away from fixed testing intervals and towards performance-oriented, risk-based monitoring.
The requirements of the standard are met by a systematically structured monitoring plan in which critical parameters such as volume flow, differential pressure and particle concentration are evaluated, monitored and incorporated into trend analyses.
This approach directly supports the Contamination Control Strategy (CCS) in accordance with Annex 1 of the EU GMP guidelines by creating a data-supported basis for controlling and minimising contamination risks.
Filters and filter integrity tests
In ventilation and clean room technology, different filter stages work together to ensure the safety and functionality of the ventilation system components and to provide a low-contamination environment. To achieve adequate filtration, different filters are often used in a single system. The classification of filters into different filter classes is specified by the ISO 16890 standard (replaces EN 779) and, for HEPA filters, by the DIN EN 1822 standard.
The integrity of HEPA filters in cleanrooms is tested to ensure that no particles enter controlled areas and compromise product quality or patient safety.
The updated DIN EN ISO 14644-3:2020-08 standard sets new requirements for leak tests, which lead to increased effort in practice. Stricter acceptance criteria, complex specifications for probe geometry and higher aerosol concentrations make implementation more difficult and place greater strain on the filters. Statistical limit values and varying scan speeds reduce the robustness of the test procedures. No simplification has been achieved compared to the previous version.