Computer and software validation
Are you looking for comprehensive support in the validation of computer systems for the pharmaceutical and medical technology industry?
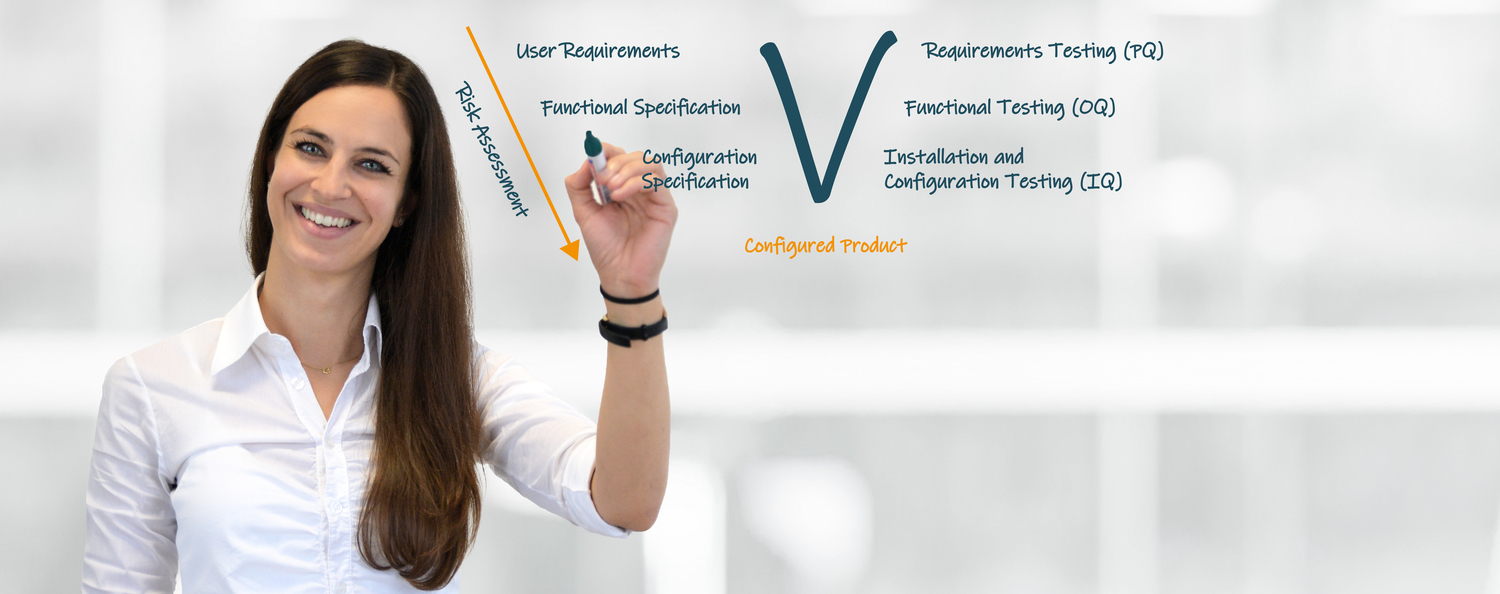
The Computer System Validation and Software Validation (CSV) ensure that procedures, processes or operations of computer-based systems reproducibly lead to the desired results. Our experts support you in the validation of your computer and software systems requirements in the pharmaceutical industry and medical technology. Together with you, we create a software matrix and determine the validation relevance of the software used in your company. Finally, the validation of your systems is carried out according to a risk-based validation approach in accordance with the requirements of GAMP 5®.
Our service for your Computer System Validation and Software Validation (CSV)
We have extensive experience in the use of computerised systems for pharmaceuticals and medical devices and understand the associated processes in quality assurance, production and quality control.
Our services therefore cover all relevant aspects of computer and software validation in accordance with GAMP 5®, Annex 11 and 21 CFR Part 11, ISO 13485 and others.
Our services in detail
- Consultancy and planning:
We help you to define the requirements and scope of your validation project and create a customised validation plan. - Project implementation:
We implement projects for you. We take care of all communication with suppliers and the associated documentation. - Risk assessment and management:
We systematically assess risks over the entire life cycle and develop strategies to minimise them. This applies in particular to risks that jeopardise data integrity. - Implementation of validation tests:
Our experts implement validation measures and carry out the necessary user and acceptance tests to ensure that your systems comply with regulatory requirements. - Documentation and reporting:
We create customised validation documentation that meets the requirements of the regulatory authorities and is highly accepted during inspections and audits. - Training and support:
We offer training for your team and provide advice and support throughout the validation process.
- Validation according to GAMP 5® of new and existing systems
- Validation of software according to ISO 13485
- Creation of validation strategies, plans and reports
- Creation and review of life cycle documents
- Creation of risk analyses / risk assessment (e.g. FMEA for the software and the process)
- Creation and review of work instructions, templates, test plans
- Periodic review
- Implementation of IQ/OQ/PQ
- Consultancy on validation strategy and documentation
- Compliance Assessments (21 CFR Part 11 / Annex 11)
- Data Integrity Assessment
- Design of validation strategy including specification documents such as SOPs, document templates, qualification/validation templates for all software categories (GAMP 5®)
- Development of a process and risk-based approach
- Basic seminars on CSV
- Validation for non-GMP users
- Production plants
- Autoclaves
- LIMS
- Test equipment management
- document management
- Deviation management
- change management
- Complaint processing
- Process control centres
- Monitoring systems
- Filling systems
- Laboratory equipment
- MES/SCADA systems
- Spreadsheets (e.g. Excel validation)
- Warehouse management systems
- PLC-controlled systems
- PC-based systems
- Client / server systems
Why choose us
- Holistic approach
We view CSV from the entire compliance perspective and consider not only the software functions, but also the requirements for processes, e.g. in production and quality control. - Digitisation expertise
We offer pharmacists support in the digitisation of processes in quality control and production and accompany them on the path to paperless documentation and production. - Full-service provider
As a full-service provider, we take on all CSV tasks in your overall project - from technical support to project management and conceptualisation. - Technical support and consulting
In addition to validation, we also offer consulting and seminar services for software validation, customised to your individual requirements. - Comprehensive expertise
Our expertise ranges from the qualification of individual computerised devices to complex validation projects for your software systems. - Customised implementation
We don't just approach projects with standard solutions, but develop flexible solutions that are tailored to the specific needs of your company.
How we understand service
- Flexibility
Thanks to our powerful team of experts, we are able to respond quickly and flexibly to your requirements for successful computerised system validation. - Pragmatic solutions
We offer practical solutions for implementing complex specifications and standards. - Wide range of services
‘We provide everything’ from system setup to document templates - customised to company size and requirements.